In the world of medical device manufacturing, precision and accuracy are non-negotiable. So, ensuring your products reach the right hands at the right time is paramount, and this all begins with the humble medical device label (barcode). Although there are many common challenges regarding UDI Label Verification, in this blog post, we'll explore the critical role medical device labels play in many industries and how understanding them can make all the difference in the world.
Skip to a Section
The Importance of the Barcode: Your Product's Integrity | What Makes Up a UDI Medical Device Label? | Not All Medical Device Labels Are Treated Equal | The Summary
The Importance of the Barcode: Your Product's Integrity
Picture This: You've just manufactured a batch of life-saving products meticulously crafted to meet the highest quality standards. These products are ready to embark on their journey to healthcare facilities around the globe. But here's the catch— at this stage, the only important thing is the integrity of the medical device label once it leaves your facility.
Yes, you read right. Your medical device labels are not just labels; they are your products. The integrity and accuracy of these medical device labels are paramount because they are the key to ensuring the right product is delivered to the right place. Consider medical device labels as the language your barcode readers understand to identify your product. If this language is flawed or unreadable, your product might as well be lost in translation.
What Does a Medical Device Label Need to Include?
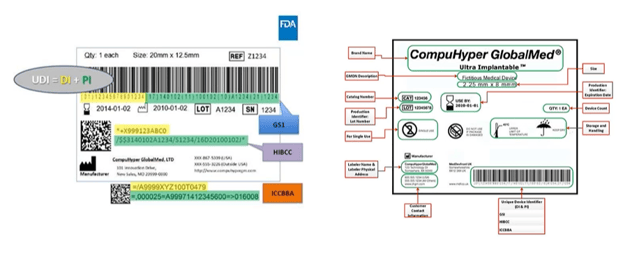
When you examine a UDI (Unique Device Identification) medical device label, you'll notice two key components: the Device Identifier (DI), which consists of numbers, and the Production Identifier (PI), which is alphabetical.
Review the list below for an overview of what these identifiers need to include, and for an even more in-depth dive, watch our free on-demand webinar on UDI labeling.
Taking a closer look
1. Device Identifier (DI) - Mandatory, static, primary access key to GUDID, identifies one or more of the following: - Labeler/manufacturer - Specific version or model device 2. Product Identifier (PI) - Conditional, variable, identifies one or more of the following: - Manufacturer lot number or batch number for the device - Serial number for the specific device - Expiration date for the device - Manufactured date of a specific device - Distinct identification code in the case of human cells, tissue, or cellular and tissue-based product (HCT/P) regulated as a device
Medical Device Label Examples |
✉️ To better grasp the significance of these components and the label reading process, consider them as street addresses. Imagine you've penned a letter. To ensure your letter reaches its intended destination, you must precisely write the address on the envelope: Building/house number first, followed by the street name, city, state, and postal code. This sequential arrangement is critical; otherwise, your letter might end up in the wrong place.

The same principle applies to your medical device UDI labels. They must be printed in a specific order to accurately convey essential information about the product and its destination. In essence, these medical device labels serve as the "street addresses” for your products in the world of medical device manufacturing.
Not All Medical Device Labels Are Treated Equal
It's important to note that not all medical device labels are used similarly. The regulations governing medical device labeling usage vary depending on the application and industry. However, regardless of the specifics, one universal truth remains: your label is your product. If the end user cannot effectively use or read your label, it ceases to be a functional product. Additionally, each packaging layer for your product, from the item level to the pallet and everything in-between, must be consistently labeled according to the FDA's UDI standards.
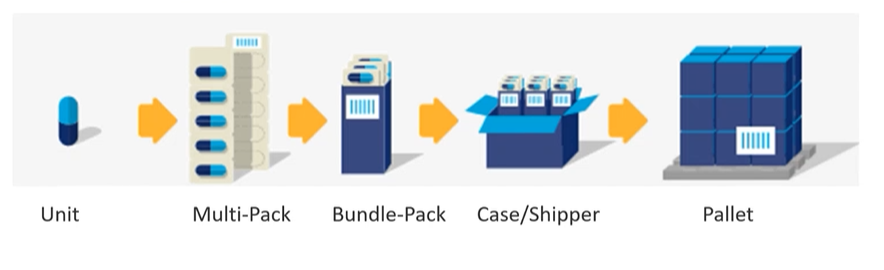
UDI labels must be present on all layers of packaging and shipping
In this fast-paced world of medical device manufacturing, where precision, accuracy, and regulatory compliance are paramount, knowledge is your most powerful tool. For an in-depth understanding of UDI Label Verification, check out our on-demand webinar to ensure your medical device labeling processes are not just up to par, but excel in their adherence to industry standards.
Remember, when it comes to medical device labeling, your barcode is not just a label—it's the lifeline connecting your product to its intended purpose and destination. Ensuring its integrity is a commitment to safety, quality, and patient well-being.
More Resources
- ISO standards
- UDI standards
- GS1/HIBCC labeling requirements
- Shop Omron Barcode Scanners & Readers
- Contact Us









Leave Comment